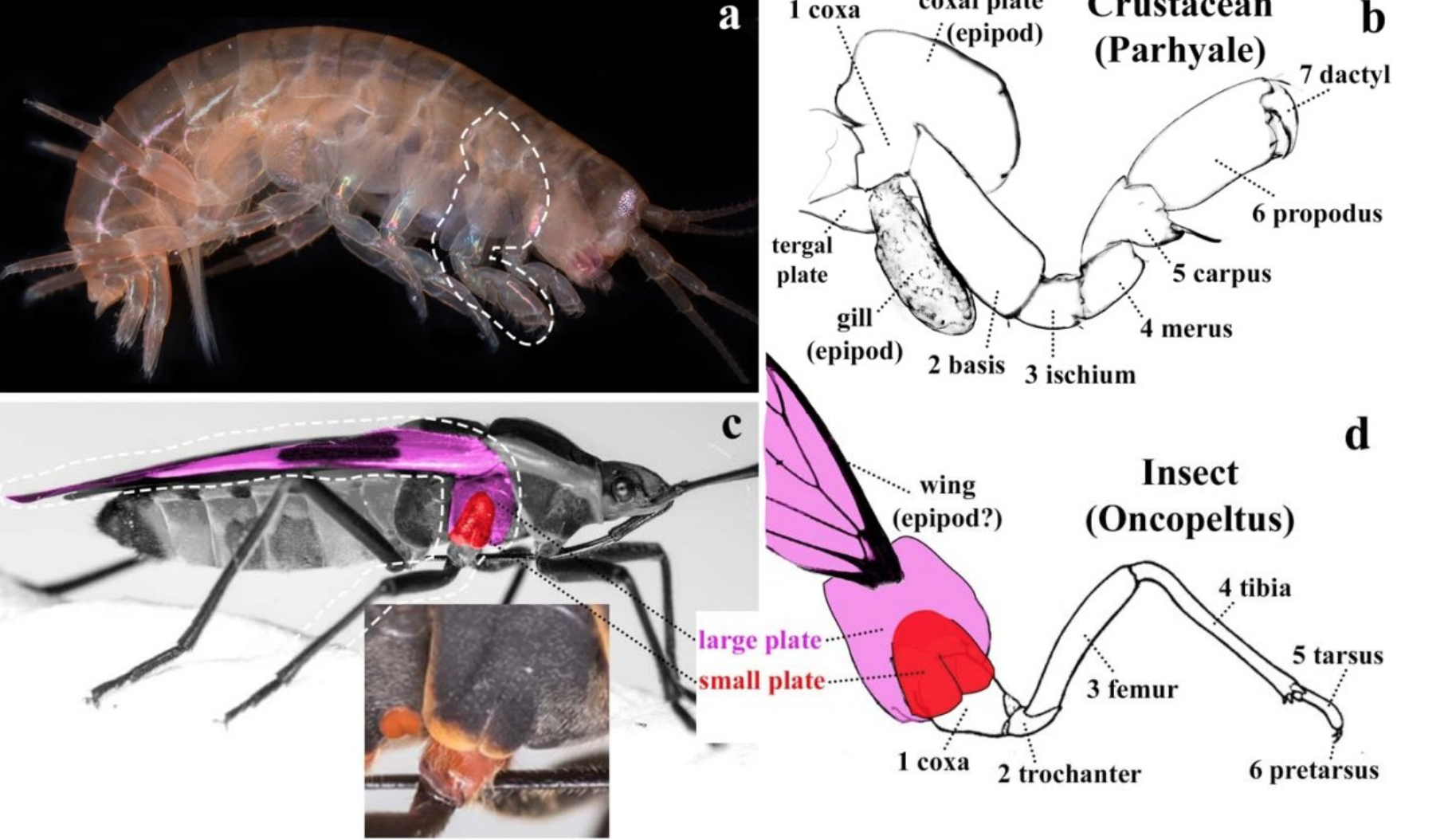
Our lab’s work on using CRISPR-Cas9 mutagenesis to explore the function of Hox genes in crustaceans was featured in a Scientific American article about the revolution in genome editing technologies. (There’s also a shout-out to CRISPR in butterflies, too!)
Nipam, as well as recent Integrative Biology PhD recipient, Erin Jarvis (now at RUMI), are quoted in the article.
Now it costs them less than $100 to knock out a gene. “Suddenly, with CRISPR, you don’t have to decide, ‘Which one gene do I want to put all my resources into?’” Jarvis says, “You can try a lot of different genes.” And with CRISPR, they’re able to break genes in up to 75 percent of Parhyaleembryos, versus a maximum 25 percent success rate with the old technique. Even better, they now have the ability to mutate several genes at once with CRISPR, which means they can now see how genes interact. When the researchers had tried to mutate multiple genes with their old technique, it rarely worked. (Though Patel notes that the older RNA technique is still very useful for certain applications).
Their research takes less time with CRISPR, too—in a study Patel’s lab published in Current Biology in 2015, they knocked out six Hox genes in about a year. Before that, they had already spent years trying to break a specific Hox gene with their old method, but were never able to do it. “Everything just goes faster,” says Patel, a professor of genetics, genomics and development. “CRISPR-Cas9 is an incredibly elegant system, and it’s very easy to control.” It also makes it simpler to study more exotic creatures (beyond the standard flies and mice), such as animals like Parhyale or butterflies. “It’s always been hard to work with a new organism,” says Jarvis, “CRISPR is awesome because suddenly, you don’t have to spend decades developing a model.” As long as you have the sequence of the gene you want to target, you’re set.
Congrats, Nipam and Erin!